Pure Water Occasional, June 14, 2019 |
Current water news is running over with stories about flooding and PFAS contamination. And, of course, National Garden Hose Day.
The persistent news: Flooding everywhere. One news cycle reported floods in Chicago, Missouri and other Arkansas River locations, Lake Ontario, Chicago, and Hungary.
The New York Times, in fact, reported that America's rivers are paralyzed because there is too much water in them.
Alaska is going to ship water into the town of Yakutat in SE Alaska because the town's 14 water wells are contaminated with PFAS. Perfluoroalkyl and polyfluoroalkyl substances have leached into area groundwater because firefighting foam required by the Federal Aviation Administration was used during practice drills at state-owned airports.
A study finds cities that experience long-term population decline are at risk for developing drinking water problems. Failing infrastructure due to a diminished tax base contributes, but the big issue is that with fewer users and more vacant buildings water stays in pipes longer, adding to problems like bacterial growth, corrosion, leaching of metals from pipes, and even increased nitrates.
Water service was shut off temporarily in Myrtle Creek, Oregon when a truck accident spilled diesel fuel near the water treatment plant. The city provided free bottled water to residents.
New research from Stanford University shows that "grass is not always greener if you water it." Details.
Residents were put at risk when high levels of copper and lead were recorded in the city water system of Sandy City, Utah. In February, a winter storm caused a fluoride pump in the city’s water system to malfunction, causing a large amount of the chemical to seep into the pipes, corroding the infrastructure and releasing the lead and other metals into the water. While fluoride itself is a potent poison, it can also cause release of metals from pipes.
"Tests of tap water, military bases and industrial sites have found PFAS contamination in more than 600 locations in 43 states. Drinking water for up to 110 million Americans may be contaminated with PFAS." --Environmental Working Group, using EPA data.
Although polychlorinated biphenyls (PCBs) were banned in most manufacturing and processing 40 years ago, the contaminant still causes problems for oil collection centers and recyclers today. PCBs are still found in motor oil. PCBs are removed by carbon filtration.
A new caffeinated sparkling water called Pep Talk has come on the market.
Work has started on a reverse osmosis desalination plant that will likely be the largest in the world. It will supply water for Abu Dhabi (United Arab Emirates). In addition to desalinated water, the plant will be a major source of clean solar energy.
PFAS in the News
We're accustomed to hearing about PFAS contamination in water. Little has been revealed about PFAS in food. Here are some excerpts from a June 3 PBS report:
The Food and Drug Administration’s first broad testing of food for a worrisome class of nonstick, stain-resistant industrial compounds found substantial levels in some grocery store meats and seafood and in off-the-shelf chocolate cake, according to unreleased findings FDA researchers presented at a scientific conference in Europe.
The levels in nearly half of the meat and fish tested were double or more the only currently existing federal advisory level for any kind of the widely used manmade compounds, which are called per- and polyfluoroalkyl substances, or PFAS.
PFAS, created by DuPont in 1938 and put into use for tough nonstick cookware, now exists in an estimated 5,000 varieties. Industries use the product to keep grease, water and stains off countless consumer items, including in food packaging, carpets and couches, dental floss and outdoor gear.
The chemicals also are found in firefighting foam, which the Department of Defense calls irreplaceable in suppressing jet-fuel fires. Especially around military bases and PFAS facilities, decades of use have built up levels in water, soil and some treated sewage sludge used to fertilize non-organic food crops and feed for livestock.
|
Happy Garden Hose Day
The stately lawn of the Serpentine Sackler Gallery in London’s Kensington Gardens was for a year home to a unique sight–a sculptural fountain made of an unruly jumble of ordinary garden hoses. French artist Bertrand Lavier created the sculpture, entitled Fountain, for a yearlong installation that ended October 4, 2015. Lavier specializes in sculptures made of “found objects.”
Lavier’s piece reminds us of the grace, the versatility, and the beauty of the common garden hose as the nation celebrates National Garden Hose Day on June 18.
|
June 18 is National Garden Hose Day
by Hardly Waite, Gazette Senior Editor
|
Everyone knows that Leonardo Da Vinci invented the lawn mower and Pure Water Annie invented the water softener, but information about the origins of one of the world’s most useful devices, the humble water hose, is hard to come by.
The year is 1652. The place: Amsterdam.
A 12-year old boy, named Jan Van der Heiden, watches in awe as the city’s town hall burns to the ground. The event makes a lasting impression.
Twenty years pass. Van der Heiden and a group of men, standing on ladders along a canal, fill a watersack which is supported in a trestle, with buckets. From the trestle, water flows in a linen hose down to the fire engine tank below. And the first fire hose is born.
The linen hoses are soon replaced by leather, which are hand-stitched, a trade that was common in Holland’s seafaring industry. It isn’t long, though before more uses are found for Van der Heiden’s invention and the first garden hose is born.
There is also talk that in the pre-Christian era, as early as 400 BC, people were using animal intestines as primitive hoses to move water about.
There is no mention of garden hoses in the Bible. I have a theory that hollowed out snakes were also among the early hoses, but these may not have been widely used until the late Middle Ages.
What is certain is that by the nineteenth century, water hoses were in use. The “hose bath” was a popular item in pre-Civil War Water Cure facilities, as indicated by this 19th century magazine picture:
When uses of the hose are discussed, the fire hose usually tops the list, and it’s true that fire fighters’ effectiveness increased exponentially when they gained the ability to get water from Place A (the water source) to Place B (the fire) without having to resort to the bucket brigade.
The common garden hose is one of those things we take for granted. The hose is pretty amazing, though, when you think of it for what it is–a very inexpensive portable pipe that can bend around corners, roll up for storage, and carry high volumes of water quickly over great distances.

Garden Hose Day, August 3, 2012. Minneapolis fans cheer on their favorites at the popular Garden Hose Pull.
|
PFAS Producers Face Increasing Legal Onslaught
The Associated Press reports a landslide of litigation directed at companies responsible for the nation’s PFAS crisis:
|
Residents of a small Delaware town, Blades, whose water supply is contaminated by chemicals linked to health issues ranging from cancer to infertility are suing several companies who manufactured the chemicals.
The News Journal of Wilmington reports the five Blades residents say they have high blood levels of perfluoroalkyl and polyfluoroalkyl substances, also called PFAS. Their lawsuit seeks to become certified as a class-action against a defunct metal plating company, 3M, DuPont and Chemours.
This lawsuit’s just one of many against the latter three companies by states including New York and New Hampshire, as well as smaller scale litigations. DuPont and Chemours agreed last year to settle lawsuits in West Virginia for $670 million. U.S. Sen. Tom Carper asked Congress this week to pass bills seeking to regulate and classify PFAS.
|
World’s rivers are contaminated with dangerous levels of antibiotics, study finds
Scientists detect drugs in two-thirds of waterways as part of first ever global study
by Phoebe Weston
|
Rivers around the world are contaminated with dangerous levels of antibiotics, according to a major new study.
Concentrations of antibiotics in some waterways exceed safe levels by 300 times, a global team of scientists led by the University of York found.
The Thames was contaminated with five antibiotics, including levels of ciprofloxacin – used to treat skin and urinary tract infections – that were three times what is considered safe.
Researchers looked at 14 commonly used antibiotics in rivers flowing through 72 countries and found antibiotics were in two-thirds of samples.
Scientists fear antibiotics in rivers cause bacteria to develop resistance meaning they can no longer be used in medicines for humans. The UN estimates that the rise in antibiotic resistance could kill 10 million people by 2050.
“A lot of the resistance genes we see in human pathogens originated from environmental bacteria,” Professor William Gaze, a microbial ecologist at the University of Exeter who was not involved in the study, told The Guardian.
Drugs get into rivers via human and animal waste, as well as leaks from wastewater treatment and drug manufacturing sources.
In one site in Bangladesh, levels of metronidazole – which is used to treat mouth and skin infections – were 300 times greater than what is considered safe. The most common antibiotic was a urinary tract infection antibiotic called trimethoprim, which was present in 307 of 711 sites tested.
Scientists flew out 92 testing kits to partners across the world who took samples from local rivers. Researchers found Bangladesh, Kenya, Ghana, Pakistan and Nigeria were home to the most contaminated rivers. The team said that the safe limits were most frequently exceeded in Asia and Africa.
However, sites in Europe, North America and South America also had high levels of contamination showing that antibiotic contamination was a “global problem”.
Professor Alistair Boxall, from the York Environmental Sustainability Institute, said:
“The results are quite eye opening and worrying, demonstrating the widespread contamination of river systems around the world with antibiotic compounds.
“Many scientists and policy makers now recognise the role of the natural environment in the antimicrobial resistance problem. Our data show that antibiotic contamination of rivers could be an important contributor.
“Solving the problem is going to be a mammoth challenge and will need investment in infrastructure for waste and wastewater treatment, tighter regulation and the cleaning up of already contaminated sites.” Professor John Wilkinson, from the University of York, said: “Until now, the majority of environmental monitoring work for antibiotics has been done in Europe, North America and China. Often on only a handful of antibiotics. We know very little about the scale of the problem globally.
“Our study helps fill this key knowledge gap with data being generated for countries that had never been monitored before.”
The findings will be unveiled at the annual meeting of the Society of Environmental Toxicology and Chemistry in Helsinki on 27 and 28 May.
|
Common Protozoa that Infect Drinking Water and How to Get Rid of Them
by Gazette Technical Wizard Pure Water Annie
|
The the most common protozoa that affect drinking water quality in the United States are Cryptospridium and Giardia. Both are intestinal parasites of warm-blooded animals. There are several species of each, and some can infect humans. Infection can come from recreational waters, drinking water, or food.
According to one authority, “Infection requires ingestion of about one to 10 organisms. Some infections are asymptomatic, so some people are not aware they are infected. Symptoms can include diarrhea and sometimes nausea, vomiting and fever. The infections are usually self-limiting, lasting several days for healthy people, but they can be chronic or fatal for less healthy or immunocompromised people.”
Both Cryptosporidium and Giardia thrive in cold water, but Crypto cysts are sensitive to higher water temperature and can survive only about a week in 85 degree Fahrenheit water. Both cysts are much more resistant to the usual water disinfectants than bacteria, and Cryptosporidium is virtually unaffected by regular municipal chlorination. Ozone and ultraviolet treatment are very effective for both, and because both cysts are relatively large, tight filtration devices like ultrafiltration, microfiltration, nanofiltration, and reverse osmosis easily remove them. In fact, a common treatment for both is conventional filtration in the two-micron range and tighter.
In a word, both cysts are fairly easily eliminated by point of use treatment like tight undersink filters and reverse osmosis and point of entry treatment by ultraviolet, but regular city water disinfection with chlorine and chloramine cannot be relied upon.
The size of the Giardia pictured above shows that the cysts are easily controlled by tight filtration devices like reverse osmosis and even conventional 1-micron filters.
|
Occasional Classics:
Some of our favorite pieces from past issues
|
 |
|
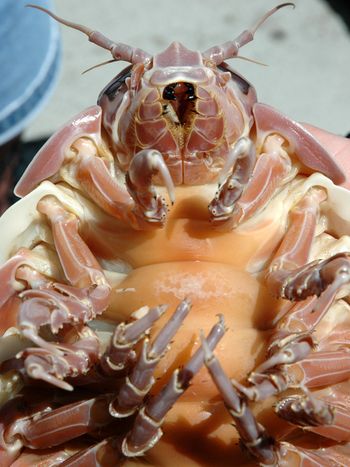
Riddle: What has seven pairs of legs and four sets of jaws, is two feet long, and has not eaten for four years? Find out. |
Aeration Is a Green and Inexpensive Treatment for Common Well Water Problems
Iron, manganese, and hydrogen sulfide are common problems with well water. There are a variety of ways to treat them, but most involve using an “oxidizer” to prepare the targeted contaminant for removal by a filter. Chlorine, potassium permanganate, and hydrogen peroxide are often used as oxidizers, but one of the very best, the least expensive, and “greenest” is plain old air. Air can be applied to well water problems in a variety of ways. Read on for details.
|
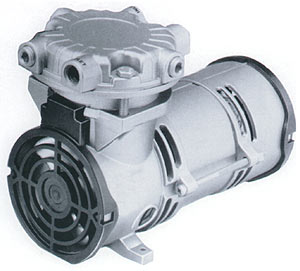 |
| Small air pump used to provide compressed air to oxidize water contaminants like iron and hydrogen sulfide. Modern air pumps are quiet, efficient, and inexpensive to operate. They provide chemical-free treatment for problem well water contaminants. More about aeration systems. |
Hardness. What Does It Mean?
Hardness in water is determined by the mineral content, specifically the amount of calcium and magnesium salts. Salts are formed when calcium and magnesium combine with bicarbonates, sulfates, chlorides, and nitrates. Hardness is a serious problem for homes and businesses because, among other problems, it forms damaging mineral deposits called scale to build up in pipes and appliances, shortening their lifespan and wasting energy.
The standard measurement of hardness in water is “grains per gallon,” with a grain representing 17.1 parts per million. Grains per gallon is usually reported “as calcium carbonate” (CaCO3) to facilitate comparison with other constituents of the water.
There are a variety of definitions of where “hard” water begins, but there is general agreement that water of 7 grains per gallon or more is hard enough that things would be improved by treatment.
Water Treatment for Hardness
The standard treatment for hardness is a water softener. It can treat up to about 100 grains per gallon of hardness, although it is less effective when a large amount of sodium is present. A softener is not a filter but an ion exchanger. It exchanges sodium for the hardness minerals, calcium and magnesium.
Reverse osmosis also removes hardness, but reverse osmosis membranes are susceptible to scale, so using RO as a softening device is usually not practical. Municipalities use a process called “lime softening” which lowers but won't remove hardness completely. Other alternatives to conventional softening include sequestering with polyphosphates and electronic- or media-based treatments that do not remove hardness but condition it to be less objectionable. Currently, TAC (Template Assisted Crystallization) technology is becoming the most commonly used alternative to conventional softening.
|
What Kind of Carbon Is Best?
or, How Is Filter Carbon Like a Parking Lot?
by Emily McBroom and Gene Franks
|
The “carbon” (often called “charcoal”) that is used for water treatment is made from a variety of raw materials. Someone has said that filter carbon can be made from anything that contains carbon, even peanut butter. Most filter carbon is made from coal–bituminous, sub-bituminous, lignite–and from nut shells, especially coconut shells.
Some of the characteristics that are considered by filter makers when choosing raw materials for the carbon products are:
- Surface area – square meters of surface per gram of carbon. The surface area determines how much adsorption can take place and what types of contaminants the carbon can take onto its surface.
- Iodine Number – indicates the ability of the carbon to adsorb small, low molecular weight organic molecules, like volatile organic chemicals.
- Molasses Number – indicates the ability of the carbon to adsorb large, high molecular weight organic molecules, like colors.
- Bulk Density – indicates the density as pounds per square foot in a column. In general, the higher the density, the more surface area available for adsorption.
Water Quality Association training materials provide such a good explanation of how these four parameters apply to carbon suitability that we can’t resist borrowing it.
The inside surface of the activated carbon particle can be viewed as a large parking lot for organic molecules. Further, one can view the large molecules as semitrucks, and the small organic molecules as compact cars. Using this viewpoint, it is easy to illustrate a number of things. First, if most of the pores in the activated carbon are micropores (small parking spaces), the semitrucks are going to have a difficult time moving inside the parking lot, and they will have difficulty finding a parking site which fits. But, the compact cars will have an easy time. (This corresponds to a high iodine number.) Second, if the pores are mostly macropores (large parking spaces), the semitrucks will be able to get around fine, but it will be an extremely inefficient way to park compact cars. (This corresponds to a high molasses number.) Third, if there are only a few roads connecting the various areas inside the parking lot, the cars will all pile up, and the roads will act as a bottleneck. Ultimately, a large number of small cars can be parked, but the parking lot will fill slowly. This is what happens if there is not a suitable mix of micropores (small spaces) and macropores (big spaces).
So, activated carbons made from lignite coal tend to have large pores (macropores) and make good parking spaces for big trucks, like tannins.
Carbons made from coconut shells have very small pores (micropores) and are especially good parking spaces for very small molecules like VOCs, which are the compact cars of the organic chemical world.
But over the years, the most widely used carbon material of all is bituminous coal, because bituminous carbon has big pores and little pores and a lot of mid-sized pores (mesopores) that are just right for parking the great many average-sized family sedans, SUVs, and pickups. In other words, bituminous carbon is widely used because it works pretty well for just about anything. Bituminous coal based activated carbons are frequently a good first choice for general dechlorination and reducing the concentration of a large range of organics.
All carbons, by the way, work well for removing chlorine and even chloramine, although contact time with the carbon needs to be about twice as long for chloramine as for chlorine. (Specially processed carbon called “catalytic carbon,” which is available in coal- or coconut-based, is much better at chloramine removal than standard carbon.) All carbons work well for taste/odor improvement, and we find no scientific basis to support the common belief that coconut shell carbons make water taste better than other carbons.
There are other considerations, of course, that are left out of the parking lot method for choosing carbon. An important one for residential users is a test called Ball-Pan Hardness. It puts a numerical value on the hardness of the carbon–how much banging around it will take before it breaks down. In this test coconut shell carbon always comes out way ahead of bituminous. This is significant for tank-style residential filters because when carbon breaks down because of the rolling and tumbling of repeated backwashing it gets into service lines. Think of it as the coconut shell parking lot having tougher walls and posts to withstand the banging it gets from those wild compact car drivers.
Carbon made from peanut butter, by the way, fares poorly on the Ball-Pan Hardness test but has an excellent Molasses number and great Surface Area.
|
Places to visit for additional information:
|
Thanks for reading and be sure to check out the next Occasional!
|
|
|
|  | |













